Beeswax

Come back later
Beeswax is a natural wax produced in the bee hive of honey bees of the genus Apis. Worker bees (the females) have eight wax-producing mirror glands on the inner sides of the sternites (the ventral shield or plate of each segment of the body) on abdominal segments 4 to 7. The size of these wax glands depends on the age of the worker and after daily flights begin these glands gradually atrophy. The new wax scales are initially glass-clear and colorless (see illustration), becoming opaque after mastication by the worker bee. The wax of honeycomb is nearly white, but becomes progressively more yellow or brown by incorporation of pollen oils and propolis. The wax scales are about 3 millimetres (0.12 in) across and 0.1 millimetres (0.0039 in) thick, and about 1100 are required to make a gram of wax.[1] Typically, for a honey bee keeper, 10 pounds of honey yields 1 pound of wax.[2]
Western honey bees use the beeswax to build honeycomb cells in which their young are raised and honey and pollen are stored. For the wax-making bees to secrete wax, the ambient temperature in the hive has to be 33 to 36 °C (91 to 97 °F). To produce their wax, bees must consume about eight times as much honey by mass. It is estimated that bees fly 150,000 miles, roughly six times around the earth, to yield one pound of beeswax (530,000 km/kg). When beekeepers extract the honey, they cut off the wax caps from each honeycomb cell with an uncapping knife or machine. Its color varies from nearly white to brownish, but most often a shade of yellow, depending on purity and the type of flowers gathered by the bees. Wax from the brood comb of the honey bee hive tends to be darker than wax from the honeycomb. Impurities accumulate more quickly in the brood comb. Due to the impurities, the wax has to be rendered before further use. The leftovers are called slumgum.
The wax may further be clarified by heating in water and may then be used for candles or as a lubricant for drawers and windows or as a wood polish. As with petroleum waxes, it may be softened by dilution with vegetable oil to make it more workable at room temperature.
Beeswax is used commercially to make fine candles, cosmetics and pharmaceuticals including bone wax (cosmetics and pharmaceuticals account for 60% of total consumption), in polishing materials (particularly shoe polish and furniture polish) and as a component of modelling waxes.
Beeswax candles are preferred in most Eastern Orthodox churches because they burn cleanly, with little or no wax dripping down the sides and little visible smoke. Beeswax is also prescribed as the material (or at least a significant part of the material) for the Paschal candle ("Easter Candle") and is recommended for other candles used in the liturgy of the Roman Catholic Church.
The burning characteristics of beeswax candles differ from those of paraffin. A beeswax candle flame has a "warmer," more yellow color than that of paraffin, and the color of the flame may vary depending on the season in which the wax was harvested.
.....Beeswax is a tough wax formed from a mixture of several compounds.
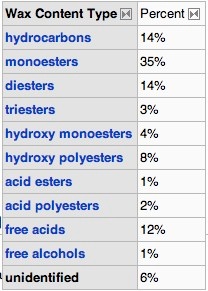
The empirical formula for beeswax is C15H31COOC30H61. Its main components are palmitate, palmitoleate, hydroxypalmitate and oleate esters of long-chain (30-32 carbons) aliphatic alcohols, with the ratio of triacontanylpalmitate CH3(CH2)29O-CO-(CH2)14CH3
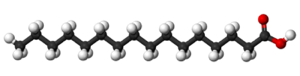
to cerotic acid CH3(CH2)24COOH, the two principal components, being 6:1.
Beeswax has a high melting point range, of 62 to 64 °C (144 to 147 °F). If beeswax is heated above 85 °C (185 °F) discoloration occurs. The flash point of beeswax is 204.4 °C (399.9 °F); there is no reported autoignition temperature. Density at 15 °C is 0.958 to 0.970 g/cm³.
Beeswax can be classified generally into European and Oriental types. The ratio of saponification value is lower (3-5) for European beeswax, and higher (8-9) for Oriental types.
Back to Main Chemistry page
Back to MAIN Pysanka home page.
Back to Pysanka Index.
Search my site with Google
Under Construction