Dyeing
The Chemistry of Pysankarstvo
Dyeing
The Chemistry of Pysankarstvo

I ended up spending the better part of a day trying to figure out how pysanka dyes work. I'd never given it much thought before, but after getting several different explanations of dyeing from my pysanka group (the "micro-etching" vs. "replacement" schools, so to speak), I decided to do a bit of research. I surfed the web, read a lot, reviewed dye theory and dredged up my ancient knowledge of chemistry (WSU BA Chemistry 1979). I’m not going to claim that this is exactly how things work, but it probably will give you a pretty good idea. This is what I have come up with:
Aniline dyes, those which modern day pysanka makers use to color their eggs, are, chemically speaking, acid dyes, meaning that they are applied from an acid solution. They are, in the dissolved form, weak acids, and form anions (negatively charged ions). Most have an amine group which can shed hydrogen ions; in the case of aniline dyes, the basic structure includes a phenyl group (usually several), sidechain (R) and an amine.
H
\
N–Phenyl–R
/
H
(The structures are actually quite complex; the structure below is an actual chemical representation of Mauveine, the first aniline dye.)
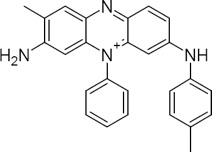
The dyes are usually sold as sodium salts which, when dissolved in water, form the active amine. Where D- is the aniline dye anion, Na+ is sodium, and H2O is water
Na2D + 2H2O –> H2D + 2NaOH
Even though the arrow is drawn in one direction, the reaction goes both ways. Compounds break up an re-form in this equilibrium state. How far in either direction this goes depends on many factors. Adding energy (heat) would push the reaction to the right (towards dissolution of the dye).
In water, H2O, the hydrogen ions are fairly tightly bound to the oxygen. In an acid, even a weak one, the hydrogen ions are less tightly bound, and would thus be more likely to react with the dye salt. This would improve dissolution and push the reaction to the right, the dissolved state, even if only ever-so-slightly.
Na2D + 2HAc –> H2D + 2NaAc
Both NaAc and NaOH are soluble in water. In solution, these compounds are in their ionic forms, as is H2D, so, more accurately, what you have is H+, OH-, Na+, Ac- and D-2 ions plus lots of molecules of H2O, forgetting hydrogen binding (which would make this discussion even more complicated).
Now that we have these loose dye ions floating around, how do we get them to bond to an egg?
First of all, we need to know exactly what it is that the dye is going to bind to. Dyes are most commonly used to color natural fibers. In textiles, acid dyes are effective on protein fibers, e.g. animal hair fibers like wool, alpaca and mohair, and silk. They are also effective in dyeing the synthetic fiber nylon, but of minimal interest in dyeing any other synthetic fibers.
Proteins are composed of amino acids. Amino acids have the basic structure
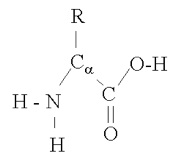








amino acid molecule
where "R" is the rest of the molecule (side-chain), α + 1 is the number of carbons in the chain, and an H has been left off of each of the carbons to simplify things a bit. As you can see, the amino acid can shed either a hydrogen ion (H) from the amine group or from the carboxyl group (OH), giving it acid and basic capabilities. Amino acids bind together in proteins in this manner , with a water molecule produced for each such bond:
H R H R
\ / \ /
N–Cα OH + N–Cα OH ––>
/ \ / / \ /
H C H C
\\ \\
O O
amino acid amino acid
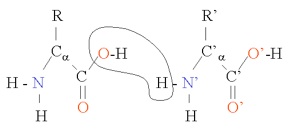








the binding of H+ and OH- to form water
H H R H R
\ \ | | /
O + N–Cα N–Cα OH
/ / \ / \ /
H H C C
\\ \\
O O
water protein (dipeptide)
Pretty, you say, but what does this have to do with aniline dyes, you may ask. Simple – the amine portion of the dye is very similar to that of the amino acid. Here in its structure of aniline, or phenylamine:
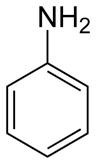








The hexagonal portion is the phenyl group; the six corners are all carbon atoms. In three dimensions, the aniline compound looks a bit like this:








This isn't germane to the conversation at hand, but it sure is a neat graphic! Aniline dyes are formed from this substrate, via complex chemical interactions that result in complex chemical formulas. For this discussion, we will simplify it and show a simplified molecule with a sidechain (R) added to one of the Cs in the ring. (To get an idea of what the dyes actually look like, chemically speaking, look here.)
So how do aniline dyes bind to proteins? One way might be a direct ionic bond between the aniline and amino acid, similar to the reaction that occurs between two amino acids. How would that look?
The amine portion (NH2) of the aniline dye could bond with amino acids (which are the components of protein) much as they bond with each other:
H R H
\ / \
N-C OH + N-Phenyl–R –––>
/ \ / /
H C H
\\
O
amino acid aniline dye
H H R H
\ \ | |
O + N–C N–Phenyl–R
/ / \ /
H H C
\\
O
water amino acid–dye complex
This binding between protein and the aniline dyes would be a direct and fairly strong one (as pictured above). But how many hydroxy (OH-) groups are available in a protein? Probably not many. But there are lots of available double-bonded oxygens. So, it is much more likely that the binding occurs via hydrogen bonds.
Hydrogen bonds are a bit weaker than direct cationic-anionic bonds, and it is thought that these are the more common bond in acid dyes. The bond occurs in chemical solutions between hydrogen and an anionic source such as oxygen, carbon or fluoride.
H – O****H – O****H H
| | \ /
H – O****H H O****H H****H H****O H
| / \ / \ / \ |
H H – O****H O O H****O – H****O – H
| |
H H
Hydrogen bonds between oxygen and hydrogen atoms occur in water, and are the main force that holds it together, are what hold water together, and keep it a liquid at fairly high temperatures. The bonds are pictured, three-dimensionally, in the illustration below. Red is the oxygen atom, and white the hydrogen. The thin green lines from the molecule in the center of the picture represent hydrogen bonds. Imagine similar lines between the other molecules of water, and you can begin to understand why water sticks together.
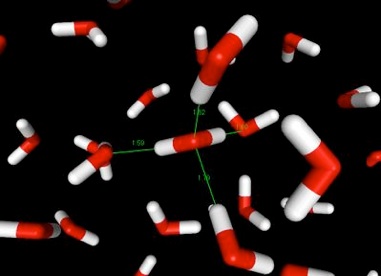








Snapshot from a simulation of liquid water.
In the protein-aniline interaction, it would be the protonated aniline reacting with the oxygen on the protein, as shown:
H R H R
\ | | /
H****N – C N – C OH protein
/ / \ / \ /
R–Phenyl–N****H C C
\ // \\
aniline H*********O O****H
\
N-Phenyl–R
/
H aniline
But what, exactly, does any of this have to do with eggs? Dyed natural fibers are protein in nature, and this is how the dyes bind. What about chicken eggs? How do the dyes bind to them?
Well, the chicken eggshell is composed of approximately 95% calcite, a crystalline form of calcium carbonate (CaCO3), and a protein matrix that binds the calcite. The shell surface is a covered by a coating of 90% protein called the cuticle. According to Wikipedia:
"Birds are known for their hard-shelled eggs. The eggshell is approximately 95% calcium carbonate crystals, which are stabilized by a protein matrix. Without the protein, the crystal structure would be too brittle to keep its form. The standard bird eggshell is a semipermeable membrane filled with thousands (1) of pores and covered on its outer surface with a cuticle, which helps the egg retain its water and keep out bacteria."
Schematic of a Chicken Egg
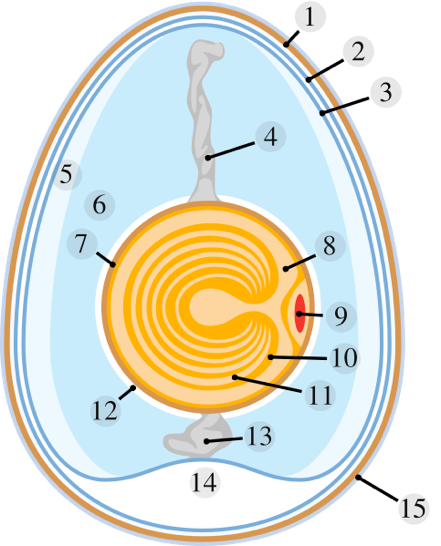
1. Eggshell 2. Outer membrane 3. Inner membrane 4. Chalaza 5. Exterior albumen (outer thin albumen)
6. Middle albumen (inner thick albumen) 7. Vitelline membrane 8. Nucleus of pander
9. Germinal disk (blastoderm) 10. Yellow yolk 11. White yolk 12. Internal albumen
13. Chalaza 14. Air cell 15. Cuticle
The weakly acidic aniline dye does not bind to the calcium carbonate in the eggshell. You can test this at home by putting a few small pieces of marble into a jar of aniline dye. Does it change color? No. Marble is just another form of calcium carbonate.
Aniline dyes actually bind to the protein cuticle. A protein, as illustrated earlier, is a series of connected amino acids; the aniline can form ionic bonds with exposed OH- groups, or hydrogen bonds to oxygen and hydrogen, just as it would to the protein structure in wool or silk. Of course, the aniline has to be protonated (acidic form, i.e. with hydrogen ions added) to do this; the more acidic a dye solution is, the more of the aniline is protonated (instead for remaining a sodium salt), and the more bonds can be formed.
So the protein cuticle is the key to it all.
A small amount of acid in the dye solution is great–it speeds up the dyeing process, and allows more dye ions to bind to the cuticle. Why don't we make the dye really acidic?
Because the acetic acid can do much more than just protonate/acidify the aniline dye and move the protein binding process forward. At greater concentrations, or with longer exposure, it can destroy the cuticle (by degrading the protein) and decalcify the eggs. You've all seen this happen when you forget you have an egg in the dye or vinegar rinse, only to discover it later––ruined!
Eggs left in dye too long will damage or lose their cuticle; you notice this when you try to dry them, and parts, or even the entire dyed surface sloughs off, leaving a much lighter egg . The protein has begun to dissolve, and the structure of the cuticle is destroyed (2). A small amount of acid over a brief period of time will help the protein binding process, but too much or for too long, and the protein itself will be degraded.
Eggs left too long in the vinegar rinse will begin to bubble, and a white powder will precipitate out, especially if you make your vinegar rinse a bit on the strong side. Why? Decalcification (3). Not only has the protective cuticle been damaged, but the actual calcium of the shell is being dissolved away. This simple (well, compared to all the others) equation says it all:
CaCO3 + 2HAc --> CaAc2 + H2O + CO2
CaCO3 is calcium carbonate (calcite), the main component of the eggshell itself, and Ac is the acetate ion CH3CO2- (making HAc acetic acid, or vinegar, in water). Mixing them together produces calcium acetate (CaAc2, the white powder), water (H2O), and carbon dioxide (CO2). Since the CO2 is a gas and bubbles out of/leaves the solution, the reaction is driven in that direction, generally until all the CaCO3 or HAc is consumed.
As you may recall, Mae West once said "Too much of a good thing is wonderful," but she wasn't talking about vinegar......









Etching, a technique used by many egg artists, takes advantage of the decalcification process to artistic effect; it is a form of controlled destruction of the egg shell.









Etching done properly after application of dyes and wax to a brown egg
And what happens when we go from one dyebath to another? When a dye and the egg are in equilibrium, most of the possible binding spots on the protein are filled. But this is not a static situation. Dyes are constantly binding and unbinding in an equilibrium situation, as hydrogen bonds are relatively weak bonds.
The egg is immersed in the new, darker dye (D). Nature wants to be in a state of equilibrium, so binding and unbinding begin again. Lighter dye molecules break their hydrogen bonds, and go into solution in the new dye. New bonds are formed with any available dye molecules but, because the D molecules now outnumber the L molecules by several orders of magnitude, it is they who form most of the new bonds. By this process, the L molecules get dissolved in the D dye, and the D molecules take over most of the binding spots on the proteins.
You can see this when you accidentally (or purposely) put an egg into a lighter dye (L) –the old, darker dye (D) will come off and get into the new dye. That's why your yellow (L) becomes slightly muddy if you put a blue (D) or green egg into it. However, the amount of D coming off is so miniscule, compared to the total number of molecules of L, that you won't notice much of a difference when you dye with L. This is all the more true for light colors put into dark ones; the few L molecules shed don't affect the D dye very much at all.
Anyway, that's my take on it, based on my reading, my knowledge of basic chemistry, and a few assumptions. Take it for what it is.
(P.S. As for dyeing etched eggs–the colors are not as bright, and can be quite muted. There is still a small amount of protein left even after etching, in the matrix that holds the calcium carbonate together in an egg shape. I can only assume it is this protein that is being dyed.)
Notes:
(1) There are tiny pores in the shells of eggs to allow the unborn animal to breathe. The domestic hen's egg has around 7500 pores.
(2) Or vinegar for that matter, but it's not as obvious to the eye in vinegar.
(3) You can also drop your marble pieces in an acid solution, and watch them dissolve. This is an identifying characteristic of marble, and the reason acid rain from air pollution is destroying so many antique Greek marble statues in Athens.
Back to Main Chemistry page
Back to MAIN Pysanka home page.
Back to Pysanka Index.
Note: All text © Luba Petrusha 2007. All Rights Reserved. Reproduction prohibited without expressed consent. May contact via e-mail (link below).
Aniline Dyes in Pysankarstvo
